Mescaline: The Chemistry and Pharmacology of its Analogs
Originally published in Lloydia
Citation: Shulgin AT. "Mescaline: The Chemistry and Pharmacology of its Analogs". Lloydia. Mar 1973;36(1):46-58.
This was presented April 24, 1972 at the Symposium on Peyote, Houston, Texas
Abstract: Although mescaline (3,4,5-trimethoxy-ß-phenethylamine, the principal alkaloid of the Peyote plant Lophophora williamsii) has been extensively explored in man as a hallucinogenic intoxicant, only a few of the natural alkaloids that accompany it in nature have been evaluated as chemical individuals. It appears that these congeners do not contribute to the reported action of the intact plant. Synthetic modifications of the structure of mescaline have been more rewarding in the search for relationships between the details of chemical structure and of biological activity. The most thoroughly investigated analogs are the substituted phenylisopropylamines. These carry the carbon skeleton of amphetamine, as is found in natural bases such at ephedrine. Ring substitution arrangements that imitate the peyote alkaloids and the phenylpropenes from the essential oils, have led to a number of hallucinogens generally more potent than mescaline. These all reflect some botanical precedent.
Other analogs are based largely on changes in the length of the carbon chain. or in the nature of the substituent in the 4-position (para to the aliphatic chain). The shortening of the chain always decreases the observed potency, but any lengthening invariably changes the pharmacological action from hallucinogenesis to relaxants or to psychic energizers. The para-substitution changes involving alkyl groups, halides, or cyclic ethers have led to a retention of mescaline-like activity, but with a consistent increase in potency.
The preceding three papers of this symposium have reviewed much of our current knowledge concerning Peyote. Dr. McLaughlin has introduced the plant with a geographic and botanical definition, and with some mention of its native usage. Dr. Kapadia has presented an up-to-date picture of the dozens of. organic compounds isolated from, or identified in it. Dr. Paul has discussed the possible pathways employed in their biosynthesis. I should like to round out this picture with a brief review of the pharmacological effects of the plant, its components, and a number of closely allied analogs, as have been reported in clinical studies in humans.
From the botanical point of view, I shall restrict this review to the single plant Lophophora williamsii (Lemaire) Coulter although note must be taken of a small residual justification for keeping the name Anhalonium lewinii alive. Although the alkaloid, mescaline, has been observed in a number of unrelated cacti, to pursue these and consequently other congeners, would lead too far away from the symposium topic, peyote.
From the chemical point of view, I intend to center this entire discussion on the parent structure of mescaline (1). Although it is of comparatively low potency, it is nonetheless one of the most dramatic drugs known from the stand point of sensory synthesis. Mescaline is probably the principle active component of the plant peyote and is certainly the best studied. This component is an easily defined and easily reproduced tool in both sociological and clinical studies.

Chemically it displays a deceptively simple structure against which the structures of the more recently studied analogs can be easily compared. From the pharmacological side, I shall limit this review to those compounds that are of known activity in man. In the large area of substituted phenethylamines related to mescaline there are literally hundreds of thousands of compounds which have been isolated from nature, or synthesized in the laboratory and described in the chemical literature. Many thousands of these have been put into one animal or another, therefore providing the raw data that have contributed to countless technical papers in the pharmaceutical and pharmacological literature. However, less than a hundred of these compounds are of known activity in man, and I shall restrict my discussion to these alone. Clinical studies in this area can be best divided into three epochs of research, Frier to the turn of the century, reports stemmed from the initial observations of Lewin both in plant description and in anthropological detail. This period also produced the earliest chemical descriptions of Heffter, and the first writings of human activity. A second period of interest flourished in the 1920's with the reports of Rouhier and Beringer describing the action of the entire plant and its principle components in man. The work of Gordon Alles, that of Hoffer and Smythies, and much of my own work has appeared over the last decade, and has concentrated to a large measure on homologs and analogs of the parent mescaline structure.
This body of pharmacological detail can be organized into three divisions: the first deals with the natural material itself and the components isolated from it which might contribute to the total effect of the intact plant; the second is a discussion of chemicals which are biosynthetically reasonable although of synthetic origin; and the third is an extension of this group to include compounds without natural precedent, but which seem to duplicate much of the physical and psychological action of mescaline.
PANPEYOTE
The first group defined above includes those materials actually found in the peyote plant. One must not only consider the entire plant, but also the extracts of the plant and the pharmacological properties which have been ascribed to the "active" components of the plant. The term panpeyote has frequently been employed for preparations in which there have been no attempts to fractionate the components of the cactus. Most of the early studies of either the peyote buttons themselves, or of the panpeyote isolates, have been reported by sociologists, anthropologists, and chemists. The medical literature is sparse and, by modern standards, unsophisticated.
The first medical report of the effects, in human subjects, of the intact peyote buttons appear to be that of Ptentiss and Morgan (1). This study employed dosages of several buttons (from three to seven per experiment) but variability of button size and potency, and of individual sensitivity to the plant, make comparisons difficult. Lewin describes the normal Indian usage of twice this number, yet recounts a thorough intoxication produced by a fraction of a single button (2). Both infusions (3) and extractions (4) of the whole plant have been evaluated. The Tincture of Peyote is an extraction of the pulverized dry plant with 70% ethanol and appears to contain all active components. This style of preparation has been employed in a number of studies (5, 6). In fact, a tincture was the form employed in the first recorded non-Indian report of medical utility (1886) (7).
Another form that has been evaluated in man is called Basic Panpeyote. This is the residue that is obtained by the evaporation of a chloroform extract of the pulverized plant. This residue contains over 30% alkaloidal material (6) and satisfactorily duplicates the human pharmacology of the intact plant (5). From such studies it was apparent that biological activity was associated with . the plant's alkaloid components, and a study of this fraction would circumvent many of the problems arising from variability of plant material. The term Soluble Peyote is a reference to an aqueous solution that results from the hack-extraction into dilute hydrochloric acid of a chloroform solution of the residue of an ammoniacal alcohol extract of the plant. This solution is also referred to as Injectable Peyote.
By the turn of the century a number of reports had appeared using one or another of these preparations, and there was a generally expressed satisfaction that both the nature and the chronology of the induced intoxication seemed to parallel that seen with the use of the plant itself. Unfortunately, most of these were concerned more with the details of the intoxication than with the composition of the intoxicant. In those reports that do concern themselves with the quantitative aspects of clinical study, it seems that when the tincture of peyote is employed, about 800 mg of alkaloidal material is required to produce full effects. With basic panpeyote, about 600 mg of alkaloids are required. All early work with both intact peyote and the various panpeyote preparations has been reviewed by Rouhier (6).
MESCALINE
With the isolation of mescaline (1) as the alkaloid principally responsible for the intoxicative action of the cactus (8) and its correct structural assignment and successful synthesis by Spath in 1919 19), the emphasis of pharmacological study shifted from the inconsistent plant to the readily synthesized compound. The pure chemical appears to duplicate most of the reported effects of the total plant in dosages compatible with its weight contribution either in the plant or in panpeyote.
At a nominally active dosage level of 350 mg, there is a generally predictable chronology of events. The first signs of change are largely physical. At about a half hour following ingestion there is an onset of nausea, often accompanied with active vomiting. There is occasionally the development of diarrhea. A mild tachycardia and slight rise in blood pressure is often seen during this initial phase, but this may be associated with anxiety and apprehension. The initial indication of sensory change is noted in about one hour. The development of central effects ends the "physical distress" phase of the intoxication, and this "sensory" phase continues to develop to a plateau of intensity during the next. two to three hours The physical changes noted during this period are minor. There is a cardiovascular quieting with the pulse rate and blood pressure dropping below their initial base levels, and a constant, extensive, but reactive, mydriasis. A gradual diminution of the central intoxication over the following few hours leads to a complete recovery generally within twelve hours. There is consistently an excellent recall of the impressions and events that occurred during the experiment.
Whereas this time pattern and sequence of events is quite predictable from one person to another and from one occasion to another, the content and direction taken by the subject's imagination as directed by his interpretive capacities are completely unpredictable and are unique to each experience. Some sensory changes are regularly noted and can be expected to contribute to the overall impact of the drug's effects. There is a shimmering and intensification of the visual field, far more intense than one might expect from the mydriasis-induced photophobia. There is an intensification of color perception, an extreme amplification of minor differences in both color and texture. Frequently observed is the generation of patterned imagery, sometimes in a grid structure, sometimes with undulating shapes, but usually with some color contribution There is a benign empathy shown to both inanimate and living things, especially to small things.
Many reviews, theses, and books have been based on the content of individual mescaline responses, and such unique anecdotal material lies outside the scope of this symposium. Reference to Beringer's studies (10) will show the range of observed responses, and Huxley (1l) has explored in depth a single experiment. This review will be restricted to general pharmacological properties, principally quantitative in nature, of the several compounds structurally related to mescaline.
An important question has as yet no satisfactory answer: To what extent does mescaline account for all of the alleged activity of peyote itself? I know of no experiment in which the two substances, the pure chemical and the total plant, have been directly compared. The pure chemical is usually assayed with a single administered dose. The plant is generally consumed over an extended period of time. The chemical is almost always investigated in a single, often isolated, subject. On the other hand the sacramental use of peyote is a group experience invoking the added variable of social interaction. The setting of a mescaline experiment is often clinical and frequently tainted with some moral reservations, whereas the peyote ceremony is an accepted ritual. Some of these factors may contribute to differences in the detail of the intoxicated state.
MESCALINE CONGENERS
Some of the suspected pharmacological differences between the pure compound mescaline and the total plant substance, may be due to any of several congeners known to be present in quantities potentially adequate to modify the intoxication syndrome. Four of the tetrahydroisoquinoline alkaloids known to be present have been evaluated in human subjects. Two of these are phenols and two are methylenedioxy ethers.

Peyotline a phenolic tetrahydroisoquinoline methylated at both the 1- and 2-positions. This compound has played an interesting role in the early research history of peyote. Heffter originally made reference to two species of peyote (13). One he called Anhalonium lewinii and although he reported the presence of mescaline and other alkaloids in it, he was unable to detect the presence of any peyotline. This variant he called "mescalinica" A second species called Anhalonium williamsii in his writing, appeared to contain only peyotline. This he referred to as "peyotlinica". The generic name of the latter variant has been modified and it has subsequently been referred to as Lophophora williamsii. This has led understandably to some confusion. It is now proper to use a single binomial for peyote (see p. 4, this issue) yet these two mutually exclusive analyses must be rationalized. A disturbing note is that while these two "variants" were morphologically indistinguishable, still they were separated into their definitional classes.
The pharmacological action of peyotline in human subjects is one of calming or sedation. rather than that of a hallucinogen. Jolly 14) has reported the production of an uneventful sleep in patients with a dosage of 50 mg. At levels of as much as 2~0 mg (total dosage) there are no indications of sensory distortions (5). There is a dizziness and a generalized tiredness that undergoes a gentle transition into sleep. This latter quantity of drug is greater than that which would be encountered in a single dosage unit of peyote. It is certainly possible that peyotline could contribute to the pharmacological picture associated with peyote as this sedative action is noted at levels that might well be encountered in the total cactus. At low levels in man i15-30 mg) there has been described a calming effect, without overt hypnosis (14).
Anhalonidine (3) is the homologue of peyotline in which the N-methyl group is absent. In man it appears to act in a manner parallel to peyotline (leading to a heavy-headedness and sedation) but with only about one-fourth the potency. At oral levels of between 100 and 250 mg there was a marked sedation, but no sensory changes whatsoever (5). It is unlikely that this compound contributes to the reported action of panpeyote.
The two remaining tetrahydroisoquinoline principles of peyote are methylenedioxy ethers rather than phenols. The first of these is lophophorine the methylenedioxy analog of peyotline. This alkaloid has been considered the most toxic of the natural components of peyote, but this claim is based solely on animal studies. In man (5) oral dosages of 20 mg led to a distinct vasodilation accompanied by an immediate headache and a warm flushed feeling. These responses are lost within the hour. The level of lophophorine found in peyote varies over a wide range and no estimate can be made concerning its contribution to the action of the entire plant, Anhalonine (5) is the methylenedioxy ether analog of anhalonidine and the N-demethyl homolog of lophophorine. This alkaloid appears to show pharmacological properties similar both quantitatively and qualitatively to its phenolic counterpart anhalonidine. A single reported experiment with 100 mg orally (5) led to an uneventful tiredness without any noticed central effects of a sensory nature.
The term "amorphous anhalonine" is occasionally encountered in the early literature on peyote. This has been shown not to be a single substance but a mixture of peyotline and lophophorine.
Five additional compounds warrant mention here, for although they are all either tract components in peyote or arguable as being possibly present on biosynthetic grounds, they have been explored in man pharmacologically. N-acetylmescaline (6) has been isolated as a trace component in the peyote plant (15) and has been identified as a metabolite of mescaline in man (16). It has been explored in acute trials in human subjects at levels between 300 and 750 mg total dosage. Only at the highest levels were any effects noticed, and they were summed up as being merely a mild degree of drowsiness (16). N-methylmescaline (7) similarly is a trace component of peyote (17). Human studies have shown no effects either peripheral or central at levels of 25 mg (18) but even this level represents many times that which would be encountered in a nominal dose of peyote.
The homologous N,-Ndimethylmescaline (trichocerine, 8) has never been observed in peyote, although it has been observed in a number of closely related cacti. It has been included in this report because of its close relationship to the well-documented presence of the mono-methyl homolog, and the known presence of methylating enzymes in the peyote plant. The compound has been found devoid of any central activity in humans even following parentetaly administered dosages of more than 500 mg (19).
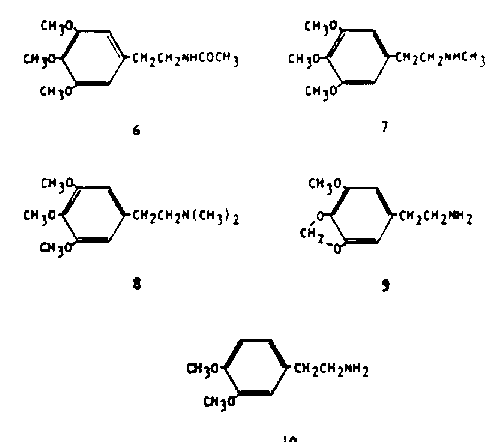
A fourth compound is homomyristicylamine (lophophine, 9) which also has never been observed in the peyote plant, but which presents an obvious theoretical potential as a biosynthetic precursor of the tetrahydroisoquinoline alkaloids such as lophophorine and anhalonine. This compound is active in man at dosage levels of 150 to 200 mg, about twice the potency of mescaline (18). The qualitative description of its action is quite similar to that of mescaline, in that there is a peaceful elevation of mood, the generation of an euphoric state, and the enhancement of visual perception especially in the color sense. There are dissimilarities, particularly in that there is little if any nausea and there is no visual distortion. These latter differences disappear at dosages of 300 mg and there is the generation of eyes-closed imagery similar to that observed with mescaline.
Some comment is in order concerning these responses to mescaline, to homomyristicylamine, and to a number of materials to be described below. This response has been given many names. It has been called hallucinogenesis, a concept that insists that there is the generation of hallucinations. The definition of a hallucination is certainly controversial, and there are very few drugs that are generally accepted as being able to produce them. 'It has been called psychotomimesis and the drugs producing it, psychotomimetics. This term suggests that the response resembles the psychotic state, and there is scant medical support for such a stand. The term psychedelic has come to imply social virtue and sanction in the use of these drugs. Each of these terms, as with others such as psychodysleptic, is clearly biased and incomplete. A compound, 3,4-dimethoxyphenethyiamine (DMPEA. 10), has received wide attention due to its association with urine analysis of patients diagnosed as schizophrenic (20). This compound has recently been reported as a trace component of peyote (21) although it is a well established component in a number of closely related cactus species. Investigation of this chemical in humans, at levels of a full gram, failed to produce any observable disturbances (22, 23).
It thus appeals that there is a structural requirement necessary for the production in human subjects of the pharmacological properties characteristic of mescaline. This is the 3,4,5- trisubstituted phenethylanine system, as illustrated by mescaline (of natural sources) and by lophophine (unnatural, but bio- synthetically logical), both active at about 5 mg, kg in man. There are many chemicals however, compounds with structures related to mescaline through botanical analogy, that show this same psychopharmacological response but with greatly increased potency. Three classifications of these "quasi-natural" compounds may be made, based simply upon the nature of the chemical structural variation. Included are those homologs that result from an extension of the aliphatic chain, the isomers that can result from the variation of the position and the number of the methoxyl groups present. and finally those analogs that result from the substitution of a methylenedioxyheterocyclic ring for two adjacent methoxyl groups.
MESCALINE: HOMOLOGS
The aliphatic chain that lies between the aromatic ring system, and the basic nitrogen, for almost all alkaloids in the plant kingdom, is two carbons long. The phenethylamine or indolethylamine (Tryptamine) system is a foundation of nearly all known pharmaceutical agents that are based upon alkaloids. The monomethylene or trimethylene homologs that have been prepared have to a large measure proved to be uninteresting pharmacologically. The most rewarding studies have come from chain lengthenings that have involved homologous families in which the basic nitrogen atom (the amine group) has been maintained in the beta-position (two carbon atoms removed from the aromatic ring). These are the so-called alpha-alkyl homologs such as the phenylisoptopylamines and the phenylsec-butylamines. The simplest of these homologs, the alphamethylphenethylamines, are stimulants of both natural and man-made origins. The compound ephedrine (11) is a component of the plant material that, and is a principle alkaloid in several of the species of Ephedra.
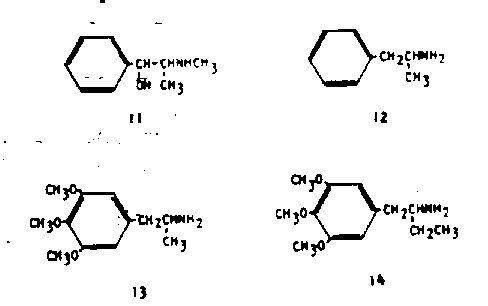
Further, this simple molecular configuration has led to the study of the completely unsubstituted analog, phenylisopropylamine (amphetamine, 12). This latter compound appears to produce a simple sympathomimetic stimulation at least at low dosages, and if there ate any "psychotomimetic" developments they are to be found only at high usage levels which can be achieved only through the development of a tolerance to the normally expected debilitating effects.
The actions of a compound that results from the combination of the features of mescaline and of amphetamine have been described (24). This compound is 3,4,5-trimethoxyamphetamine (3,4,5-trimethoxyphenylisopropylamine, TMA, 13) and it has been established as being an effective sensory distorting agent at levels of about 175 mg (25). It is thus about twice as potent as mescaline.3 An extension of this chain to the 4-carbon homolog (alpha-ethyl- mescaline, 14) produces a compound that lacks all central and peripheral activity in human subjects at acute levels as high as 220 mg (27).
These several chemicals have laid the groundwork for all of the substances to be discussed below, namely that the three carbon chain, the phenyiisopropylamine or amphetamine structure, has proved to be optimum for the generation · of these sensory distortion effects. A decrease of the chain length to two carbons (the loss of the alpha-methyl group) usually retains the nature of activity but with some decrease in potency. A lengthening of the chain to four carbons (the substitution of an alpha-ethyl group) changes the qualitative nature of the pharmacological response.
[ The carbon skeleton end positional location of the ether oxygen atom in 13 (end in MMDA. 25. below) are identical to that found in elemicin end myristicin. components of the spice nutmeg. These Latter nitrogen-free essential cils have recently been shown capable of being converted into the above mentioned amphetamine analogs under biological conditions (26).]
MESCALINE ANALOGS
Variations of the substitution pattern of the methoxyl groups within this generalization of a three-carbon chain optimum, have led to extensive changes in potency. Using 13 as a parent structure, there are five possible positional isomers. These have been synthesized (28) and clinically evaluated in man (29). The first of these positional isomers (2,4,5-trimethoxyphenylisopropylamine, TMA-2, 15) is also related to a series of essential oils, the unconjugated 2,4,5-

trimethoxy- l-allylbenzene found in Caesulia axillaries (30) and the conjugated counterpart in a number of species of Asarum and Acorus (31). The isomer has proven to be the most potent of all six, with an effective dosage level of 20 mg, about a twentieth that required for effective mescaline intoxication. The vicinal analog (2,3,4-tiimethoxyphenylisopropylamine, TMA-3, 16) has neither natural counterparts nor central activity.
The symmetrical isomer (2,4,6-trimethoxyphenylisopropylamine, TMA-6, 17) is without known analogy in the essential oils, but this ring substitution pattern is extremely common throughout the many plant products related to the chromones and the flavonoids. The styles of central activity of 15 and If ate similar to one another in that at threshold levels there is noted only an enjoyable light-headedness coupled with distinct euphoria and a minimum of perceptive distortion. At effective dosages there again is the nausea reminiscent of that seen with mescaline, and the visual distortions can become quite extensive. The sensory disturbances and syntheses so often found to be entertaining or instructive in the case of mescaline intoxication, are found to be disturbing with these latter drugs. TMA-6, If, is about half as potent as 15, thus having some ten times the effectiveness of mescaline. The two remaining possible isomers are known, have been titrated clinically to determine their relative potencies, but have not been explored to an extent adequate for generalizations concerning the qualitative nature of their actions. 2,3,5-Trimethoxyphenyiisopropylamine (TMA-4, 18) is an effective central intoxicant at a 90 mg dosage, and 2,3,6 -trimethoxyphenyli sopropylamine (TMA-5, 19) has similar activity at 30 mg. Both ate thus clearly more potent than mescaline,
A number of isomers of 13 have been studied wherein the variation is with the number of methoxy groups rather than with their position. A single tetramethoxy analog is known, 2,3,4,5- tetramethoxyphenylisopropylamine (20). As with others of the active phenylisopropylamines, there is a corresponding essential oil in nature. This exact carbon skeleton is found in 2,3,4,5-tetramethoxy-l-allyl benzene, one of the major constituents of the seed oil of Apium spp. (32). Compound 20 is effective in man at a dosage of 60 mg, i.e., six times as potent as mescaline (29).

Three of the six possible dimethoxyphenylisopropylamines have been explored pharmacologically. The compound most closely allied with both botanical and mammalian biochemistry is 3,4-dimethoxyphenylisopropylamine (DMA, 21). The plant correlation is to methyleugenol and methylisoeugenol, common essential oils found in a host of plants. In animal chemistry, 3,4-dihydroxyphenethylamine (dopamine) is an intermediate in the synthesis of the transmitter norepinephrine, and- 21 is its trimethyl homolog. The role and action of the two-carbon analog 10 have been discussed above. Compound 21 is slightly less potent than mescaline (33) in that levels of about 700 mg were required parenterally to produce the color syntheses and other sensory distortions ascribed to mescaline. The other two dimethoxy isomers that have been studied, 2,4-dimethoxyphenylisopropylamine (22) and 2,5-dimethoxyphenylisopropylamine (23), are considerably more potent, being active at 70 mg and 45 mg orally, resp. (29).
Only one monomethoxy analog has been explored clinically. In animal studies, this compound (4-methoxyphenylisopropylamine, PMA, 24) is reported to have an hallucinogenic potential parallel to LSD (34).. In man, however, some 75 mg is required (orally) for effective central activity, thus being closer to mescaline than LSD in potency. Arguments that this compound might be an active metabolite of amphetamine in cases of amphetamine-induced psychoses, have been answered by the reported failure to observe this compound in human urine samples following heavy amphetamine administration. It could only be found as an excretory product following its own administration to human subjects (35).
Bases that contain a methylenedioxy ring in place of two adjacent methoxyl groups constitute a large family of compounds that parallel most of the above-mentioned polymethoxylated phenylisopropylamines . In nature, this form of biosynthetic relationship is frequently seen. It occurs within the tetrahydroisoquinoiine alkaloids of peyote itself. A generalized qualitative comment can be made concerning the methylenedioxyphenylisoptopylamines itemized here. In almost every case in which a specific comparison is available between a methylenedioxy compound and its dimethoxy counterpart, the methylenedioxy com pound shows less potential for visual distortion, it is generally more controllable in its extremes of central activity and, as a rule, it is more potent. Of this family only two compounds, 25 and 33, have been sufficiently studied to warrant extended discussion of qualitative subtleties.
Just as there are six possible trimethoxy-substitution isomers of amphetamine, so there are six possible methoxymethylenedioxy isomers. Five of these six have been described chemically and pharmacologically. Their trivial names relate the position of the oxygen substituents directly to the trimethoxy counter part; i.e., MMDA-5 and TMA-5 are both 2,3,6-substitution products. The isomer that is the direct relative of mescaline (1) and the theoretical lophophine (9) is 3-methoxy-4,5-methyienedioxyphenyiisopro (MMDAI 25). The carbon skeleton in this instance is that of myristicin and isomyristicin, essential oils found throughout the plant kingdom. This compound was synthesized and evaluated independently in two separate laboratories (36, 37). The compound is active in man at 130 mg, and so has nearly three times the potency of mescaline. Unlike mescaline, however, its actions are largely free of perceptual distortions, and seem not to inflexibly command the complete attention of the subject. There is an element of choice in the degree of compliance to the intoxication, and there seems to be some valid access to memory material. These properties have been discussed in a recent monograph (38). The compound analogous to TMA-2 (15) is 2-methoxy-4,5-methylenedioxyphenylisopropylamine (MMDA-2, 26). An essential oil (carpacin) with this substitution pattern has been isolated from Cinnamomumspp. (39).
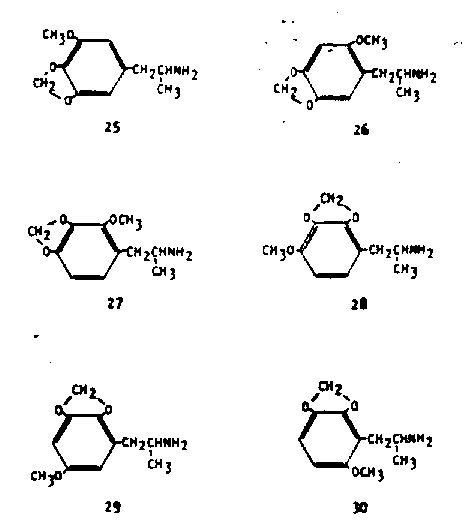
The base 26 is only slightly less active in human trials (30 mg) than the trimethoxy counterpart. Two possible 2,3,4- trisubstituted compounds can exist that correspond to 16. These are 2-methoxy-3,4-methylenedioxyphenylisopro (MMDA- 39, 21) and 4-methoxy-2,3 -methylenedioxyphenyiisopropylamine (MMDA-3b, 28). The first of these has a natural counterpart in the ether croweacin isolated from Eriostemon crowei (40); the second has no known analogy in nature. Although the trimethoxy compound with this vicinal substitution pattern was not active, both of these compounds have been shown to be effective intoxicants, the first at a 35 mg dose (41) and the second at 120 mg (29). The fifth of these methoxy methylenedioxy isomers is 6-mtthoxy-2,3-methylentdioxyphenylisopropylamine (MMDA-5, 30) which corresponds exactly to TMA-5 (19) both in substitution pattern and in potency (42). No successful synthesis yet exists for 5-methoxy-2,3-methylenedioxvphenylisopro (MMDA-4, 29). The analog to TMA-6 (lit) cannot of course exist. Only three methylenedioxy compounds that are not trisubstituted have beer studied in man. Two of these are the tetrasubstituted analogs of apiole and dill-apiole, both well explored essential oils from a number of spices.

These are 2,5 -dimethoxy-3,4-methylenedioxyphenyiisopr (31) and 2,3-di-methoxy-4 ,S-methylenedioxyphenyiisopropylamine (.32). They are active at 30 mg and 70 mg resp. (43) corresponding to twelve times, and five times the potency of mescaline. The -remaining compound is 3,4-methylenedioxyphenyl isopropylamine (MDA, 33) which, along with MMDA (25) is the only material sufficiently well studied to warrant generalizations concerning comparative qualitative properties (44). Although this compound is not particularly potent (typical dosage, some 120 mg or about three times the activity of mescaline) its easy availability and apparent consistency of action have led to its study in a number of laboratories. The 2,3-isomer is unknown.
The last group to be discussed consists of compounds that are quite removed from nature. They have evolved quite logically however from the importance that is clearly assignable to the 4-position of the aromatic substitution pattern. An example is that, of all the several possible ethoxy homologs of the maximally potent trimethoxy-compound 15, it is only the 4-ethoxy compound (2,5 -dimethoxy-4-ethoxyphenylisopropylamine, MEM, 3r) that maintains complete central activity at the same potency (45). An argument can be extended that this positional sensitivity might be associated with a theoretical ease of metabolic attack at this position. Consistent with this approach, one could expect that the replacement of an easily attackable group (alkoxy) with one less amenable to removal (aikyl) might radically affect the pharmacological properties of the corresponding amine. The simplest such example, 2,5-di-methoxy-4-methylphenylisoptopylamine (DOM, STP, 35)
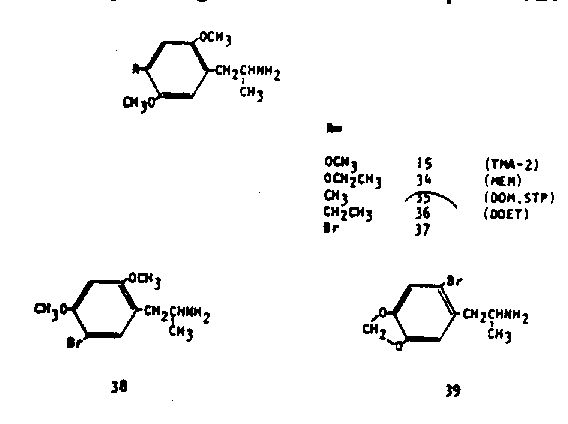
proved to be several times more potent than 15 as a centrally active psychotomimetic. With an effective, not threshold, level of activity of 3 mg total dosage, it has over one hundred times the potency of mescaline. Further, the nature and chronology of the intoxication is quite changed from that common to most of these chemicals discussed. Most noteworthy is the unexpected lengthening of the time course by a factor of three or more. Several clinical studies have appeared in the medical literature concerning this drug (46, 47). The 4-ethyl homolog (2,5-dimethoxy-4-ethyiphenylisopropylamine, DOET, 36) appears to be fully as potent as DOM (35) although at lower clinical levels the psychological changes noted seem to be somewhat more controllable and thus more constructive (118). Recently yet another analog of this system has been described. In this, the 4-methyl group has been replaced with a bromo substituent to produce 2,5dimethoxy-4-bromophenylisopropylamine (37) (49, 50). This compound has in man an effective response level of between 0.5 and 1.0 mg, thus it is several hundred times more potent than mescaline. Yet in the qualitative sense it presents more of the clinical picture that has been associated with MDA (33) (49). A positional isomer has been described (2,4-dimethoxy-5-bromophenylisopropylamine, 38) which is much less potent than 37 (100 mg for an effective intoxication) but which maintains the MDA-like response is1>. The 2-bromoanalog of MMDA-2 (2-bromo-4,5-methylenedioxy·phenylisopropolymine) is less active than mescaline (>350 mg in man) and seems to produce an amphetamine-like response.
A final comment is in order concerning the frequent use of the term "amphetamines" to describe these compounds. With the exception of some of the peyote components, all of the centrally active substances that have been discussed here contain the carbon skeleton of the amphetamine molecule. Yet almost none of them display the sympathomimetic stimulation that is the reputation of amphetamine. The popular use of the term "amphetamines" has come to embrace a large number of currently used drugs that are stimulants, and which call upon these effects for their effectiveness. Examples of this are amphetamine itself (and its dextrorotatory isomer, dexedrine), methamphetamine, methylphenidate (Ritalin), phenmetrazine (Preludin) and mephenteramine (Wyamine). An excellent demonstration of the separation of these pharmacological areas may be seen from the fact that the active isomer of DOM (35) is the R (-) form. The S (+) form shows only stimulant properties, and this is the same absolute configuration as that seen in the stimulatively active isomer of amphetamine itself, S-(+) -dextroamphetamine (52).
This has been an up-to-date presentation of our knowledge of the structural requirements for hallucinogenesis (or whatever this form of central stimulation and sensory modification might be called). At this state of chemical evolution, the structure of the parent compound mescaline might well look quite foreign. However, its role has been evident as a starting point in this rapidly evolving area of psychopharmacology.
Abstract: Although mescaline (3,4,5-trimethoxy-ß-phenethylamine, the principal alkaloid of the Peyote plant Lophophora williamsii) has been extensively explored in man as a hallucinogenic intoxicant, only a few of the natural alkaloids that accompany it in nature have been evaluated as chemical individuals. It appears that these congeners do not contribute to the reported action of the intact plant. Synthetic modifications of the structure of mescaline have been more rewarding in the search for relationships between the details of chemical structure and of biological activity. The most thoroughly investigated analogs are the substituted phenylisopropylamines. These carry the carbon skeleton of amphetamine, as is found in natural bases such at ephedrine. Ring substitution arrangements that imitate the peyote alkaloids and the phenylpropenes from the essential oils, have led to a number of hallucinogens generally more potent than mescaline. These all reflect some botanical precedent.
Other analogs are based largely on changes in the length of the carbon chain. or in the nature of the substituent in the 4-position (para to the aliphatic chain). The shortening of the chain always decreases the observed potency, but any lengthening invariably changes the pharmacological action from hallucinogenesis to relaxants or to psychic energizers. The para-substitution changes involving alkyl groups, halides, or cyclic ethers have led to a retention of mescaline-like activity, but with a consistent increase in potency.
The preceding three papers of this symposium have reviewed much of our current knowledge concerning Peyote. Dr. McLaughlin has introduced the plant with a geographic and botanical definition, and with some mention of its native usage. Dr. Kapadia has presented an up-to-date picture of the dozens of. organic compounds isolated from, or identified in it. Dr. Paul has discussed the possible pathways employed in their biosynthesis. I should like to round out this picture with a brief review of the pharmacological effects of the plant, its components, and a number of closely allied analogs, as have been reported in clinical studies in humans.
From the botanical point of view, I shall restrict this review to the single plant Lophophora williamsii (Lemaire) Coulter although note must be taken of a small residual justification for keeping the name Anhalonium lewinii alive. Although the alkaloid, mescaline, has been observed in a number of unrelated cacti, to pursue these and consequently other congeners, would lead too far away from the symposium topic, peyote.
From the chemical point of view, I intend to center this entire discussion on the parent structure of mescaline (1). Although it is of comparatively low potency, it is nonetheless one of the most dramatic drugs known from the stand point of sensory synthesis. Mescaline is probably the principle active component of the plant peyote and is certainly the best studied. This component is an easily defined and easily reproduced tool in both sociological and clinical studies.

Chemically it displays a deceptively simple structure against which the structures of the more recently studied analogs can be easily compared. From the pharmacological side, I shall limit this review to those compounds that are of known activity in man. In the large area of substituted phenethylamines related to mescaline there are literally hundreds of thousands of compounds which have been isolated from nature, or synthesized in the laboratory and described in the chemical literature. Many thousands of these have been put into one animal or another, therefore providing the raw data that have contributed to countless technical papers in the pharmaceutical and pharmacological literature. However, less than a hundred of these compounds are of known activity in man, and I shall restrict my discussion to these alone. Clinical studies in this area can be best divided into three epochs of research, Frier to the turn of the century, reports stemmed from the initial observations of Lewin both in plant description and in anthropological detail. This period also produced the earliest chemical descriptions of Heffter, and the first writings of human activity. A second period of interest flourished in the 1920's with the reports of Rouhier and Beringer describing the action of the entire plant and its principle components in man. The work of Gordon Alles, that of Hoffer and Smythies, and much of my own work has appeared over the last decade, and has concentrated to a large measure on homologs and analogs of the parent mescaline structure.
This body of pharmacological detail can be organized into three divisions: the first deals with the natural material itself and the components isolated from it which might contribute to the total effect of the intact plant; the second is a discussion of chemicals which are biosynthetically reasonable although of synthetic origin; and the third is an extension of this group to include compounds without natural precedent, but which seem to duplicate much of the physical and psychological action of mescaline.
PANPEYOTE
The first group defined above includes those materials actually found in the peyote plant. One must not only consider the entire plant, but also the extracts of the plant and the pharmacological properties which have been ascribed to the "active" components of the plant. The term panpeyote has frequently been employed for preparations in which there have been no attempts to fractionate the components of the cactus. Most of the early studies of either the peyote buttons themselves, or of the panpeyote isolates, have been reported by sociologists, anthropologists, and chemists. The medical literature is sparse and, by modern standards, unsophisticated.
The first medical report of the effects, in human subjects, of the intact peyote buttons appear to be that of Ptentiss and Morgan (1). This study employed dosages of several buttons (from three to seven per experiment) but variability of button size and potency, and of individual sensitivity to the plant, make comparisons difficult. Lewin describes the normal Indian usage of twice this number, yet recounts a thorough intoxication produced by a fraction of a single button (2). Both infusions (3) and extractions (4) of the whole plant have been evaluated. The Tincture of Peyote is an extraction of the pulverized dry plant with 70% ethanol and appears to contain all active components. This style of preparation has been employed in a number of studies (5, 6). In fact, a tincture was the form employed in the first recorded non-Indian report of medical utility (1886) (7).
Another form that has been evaluated in man is called Basic Panpeyote. This is the residue that is obtained by the evaporation of a chloroform extract of the pulverized plant. This residue contains over 30% alkaloidal material (6) and satisfactorily duplicates the human pharmacology of the intact plant (5). From such studies it was apparent that biological activity was associated with . the plant's alkaloid components, and a study of this fraction would circumvent many of the problems arising from variability of plant material. The term Soluble Peyote is a reference to an aqueous solution that results from the hack-extraction into dilute hydrochloric acid of a chloroform solution of the residue of an ammoniacal alcohol extract of the plant. This solution is also referred to as Injectable Peyote.
By the turn of the century a number of reports had appeared using one or another of these preparations, and there was a generally expressed satisfaction that both the nature and the chronology of the induced intoxication seemed to parallel that seen with the use of the plant itself. Unfortunately, most of these were concerned more with the details of the intoxication than with the composition of the intoxicant. In those reports that do concern themselves with the quantitative aspects of clinical study, it seems that when the tincture of peyote is employed, about 800 mg of alkaloidal material is required to produce full effects. With basic panpeyote, about 600 mg of alkaloids are required. All early work with both intact peyote and the various panpeyote preparations has been reviewed by Rouhier (6).
MESCALINE
With the isolation of mescaline (1) as the alkaloid principally responsible for the intoxicative action of the cactus (8) and its correct structural assignment and successful synthesis by Spath in 1919 19), the emphasis of pharmacological study shifted from the inconsistent plant to the readily synthesized compound. The pure chemical appears to duplicate most of the reported effects of the total plant in dosages compatible with its weight contribution either in the plant or in panpeyote.
At a nominally active dosage level of 350 mg, there is a generally predictable chronology of events. The first signs of change are largely physical. At about a half hour following ingestion there is an onset of nausea, often accompanied with active vomiting. There is occasionally the development of diarrhea. A mild tachycardia and slight rise in blood pressure is often seen during this initial phase, but this may be associated with anxiety and apprehension. The initial indication of sensory change is noted in about one hour. The development of central effects ends the "physical distress" phase of the intoxication, and this "sensory" phase continues to develop to a plateau of intensity during the next. two to three hours The physical changes noted during this period are minor. There is a cardiovascular quieting with the pulse rate and blood pressure dropping below their initial base levels, and a constant, extensive, but reactive, mydriasis. A gradual diminution of the central intoxication over the following few hours leads to a complete recovery generally within twelve hours. There is consistently an excellent recall of the impressions and events that occurred during the experiment.
Whereas this time pattern and sequence of events is quite predictable from one person to another and from one occasion to another, the content and direction taken by the subject's imagination as directed by his interpretive capacities are completely unpredictable and are unique to each experience. Some sensory changes are regularly noted and can be expected to contribute to the overall impact of the drug's effects. There is a shimmering and intensification of the visual field, far more intense than one might expect from the mydriasis-induced photophobia. There is an intensification of color perception, an extreme amplification of minor differences in both color and texture. Frequently observed is the generation of patterned imagery, sometimes in a grid structure, sometimes with undulating shapes, but usually with some color contribution There is a benign empathy shown to both inanimate and living things, especially to small things.
Many reviews, theses, and books have been based on the content of individual mescaline responses, and such unique anecdotal material lies outside the scope of this symposium. Reference to Beringer's studies (10) will show the range of observed responses, and Huxley (1l) has explored in depth a single experiment. This review will be restricted to general pharmacological properties, principally quantitative in nature, of the several compounds structurally related to mescaline.
An important question has as yet no satisfactory answer: To what extent does mescaline account for all of the alleged activity of peyote itself? I know of no experiment in which the two substances, the pure chemical and the total plant, have been directly compared. The pure chemical is usually assayed with a single administered dose. The plant is generally consumed over an extended period of time. The chemical is almost always investigated in a single, often isolated, subject. On the other hand the sacramental use of peyote is a group experience invoking the added variable of social interaction. The setting of a mescaline experiment is often clinical and frequently tainted with some moral reservations, whereas the peyote ceremony is an accepted ritual. Some of these factors may contribute to differences in the detail of the intoxicated state.
MESCALINE CONGENERS
Some of the suspected pharmacological differences between the pure compound mescaline and the total plant substance, may be due to any of several congeners known to be present in quantities potentially adequate to modify the intoxication syndrome. Four of the tetrahydroisoquinoline alkaloids known to be present have been evaluated in human subjects. Two of these are phenols and two are methylenedioxy ethers.

Peyotline a phenolic tetrahydroisoquinoline methylated at both the 1- and 2-positions. This compound has played an interesting role in the early research history of peyote. Heffter originally made reference to two species of peyote (13). One he called Anhalonium lewinii and although he reported the presence of mescaline and other alkaloids in it, he was unable to detect the presence of any peyotline. This variant he called "mescalinica" A second species called Anhalonium williamsii in his writing, appeared to contain only peyotline. This he referred to as "peyotlinica". The generic name of the latter variant has been modified and it has subsequently been referred to as Lophophora williamsii. This has led understandably to some confusion. It is now proper to use a single binomial for peyote (see p. 4, this issue) yet these two mutually exclusive analyses must be rationalized. A disturbing note is that while these two "variants" were morphologically indistinguishable, still they were separated into their definitional classes.
The pharmacological action of peyotline in human subjects is one of calming or sedation. rather than that of a hallucinogen. Jolly 14) has reported the production of an uneventful sleep in patients with a dosage of 50 mg. At levels of as much as 2~0 mg (total dosage) there are no indications of sensory distortions (5). There is a dizziness and a generalized tiredness that undergoes a gentle transition into sleep. This latter quantity of drug is greater than that which would be encountered in a single dosage unit of peyote. It is certainly possible that peyotline could contribute to the pharmacological picture associated with peyote as this sedative action is noted at levels that might well be encountered in the total cactus. At low levels in man i15-30 mg) there has been described a calming effect, without overt hypnosis (14).
Anhalonidine (3) is the homologue of peyotline in which the N-methyl group is absent. In man it appears to act in a manner parallel to peyotline (leading to a heavy-headedness and sedation) but with only about one-fourth the potency. At oral levels of between 100 and 250 mg there was a marked sedation, but no sensory changes whatsoever (5). It is unlikely that this compound contributes to the reported action of panpeyote.
The two remaining tetrahydroisoquinoline principles of peyote are methylenedioxy ethers rather than phenols. The first of these is lophophorine the methylenedioxy analog of peyotline. This alkaloid has been considered the most toxic of the natural components of peyote, but this claim is based solely on animal studies. In man (5) oral dosages of 20 mg led to a distinct vasodilation accompanied by an immediate headache and a warm flushed feeling. These responses are lost within the hour. The level of lophophorine found in peyote varies over a wide range and no estimate can be made concerning its contribution to the action of the entire plant, Anhalonine (5) is the methylenedioxy ether analog of anhalonidine and the N-demethyl homolog of lophophorine. This alkaloid appears to show pharmacological properties similar both quantitatively and qualitatively to its phenolic counterpart anhalonidine. A single reported experiment with 100 mg orally (5) led to an uneventful tiredness without any noticed central effects of a sensory nature.
The term "amorphous anhalonine" is occasionally encountered in the early literature on peyote. This has been shown not to be a single substance but a mixture of peyotline and lophophorine.
Five additional compounds warrant mention here, for although they are all either tract components in peyote or arguable as being possibly present on biosynthetic grounds, they have been explored in man pharmacologically. N-acetylmescaline (6) has been isolated as a trace component in the peyote plant (15) and has been identified as a metabolite of mescaline in man (16). It has been explored in acute trials in human subjects at levels between 300 and 750 mg total dosage. Only at the highest levels were any effects noticed, and they were summed up as being merely a mild degree of drowsiness (16). N-methylmescaline (7) similarly is a trace component of peyote (17). Human studies have shown no effects either peripheral or central at levels of 25 mg (18) but even this level represents many times that which would be encountered in a nominal dose of peyote.
The homologous N,-Ndimethylmescaline (trichocerine, 8) has never been observed in peyote, although it has been observed in a number of closely related cacti. It has been included in this report because of its close relationship to the well-documented presence of the mono-methyl homolog, and the known presence of methylating enzymes in the peyote plant. The compound has been found devoid of any central activity in humans even following parentetaly administered dosages of more than 500 mg (19).

A fourth compound is homomyristicylamine (lophophine, 9) which also has never been observed in the peyote plant, but which presents an obvious theoretical potential as a biosynthetic precursor of the tetrahydroisoquinoline alkaloids such as lophophorine and anhalonine. This compound is active in man at dosage levels of 150 to 200 mg, about twice the potency of mescaline (18). The qualitative description of its action is quite similar to that of mescaline, in that there is a peaceful elevation of mood, the generation of an euphoric state, and the enhancement of visual perception especially in the color sense. There are dissimilarities, particularly in that there is little if any nausea and there is no visual distortion. These latter differences disappear at dosages of 300 mg and there is the generation of eyes-closed imagery similar to that observed with mescaline.
Some comment is in order concerning these responses to mescaline, to homomyristicylamine, and to a number of materials to be described below. This response has been given many names. It has been called hallucinogenesis, a concept that insists that there is the generation of hallucinations. The definition of a hallucination is certainly controversial, and there are very few drugs that are generally accepted as being able to produce them. 'It has been called psychotomimesis and the drugs producing it, psychotomimetics. This term suggests that the response resembles the psychotic state, and there is scant medical support for such a stand. The term psychedelic has come to imply social virtue and sanction in the use of these drugs. Each of these terms, as with others such as psychodysleptic, is clearly biased and incomplete. A compound, 3,4-dimethoxyphenethyiamine (DMPEA. 10), has received wide attention due to its association with urine analysis of patients diagnosed as schizophrenic (20). This compound has recently been reported as a trace component of peyote (21) although it is a well established component in a number of closely related cactus species. Investigation of this chemical in humans, at levels of a full gram, failed to produce any observable disturbances (22, 23).
It thus appeals that there is a structural requirement necessary for the production in human subjects of the pharmacological properties characteristic of mescaline. This is the 3,4,5- trisubstituted phenethylanine system, as illustrated by mescaline (of natural sources) and by lophophine (unnatural, but bio- synthetically logical), both active at about 5 mg, kg in man. There are many chemicals however, compounds with structures related to mescaline through botanical analogy, that show this same psychopharmacological response but with greatly increased potency. Three classifications of these "quasi-natural" compounds may be made, based simply upon the nature of the chemical structural variation. Included are those homologs that result from an extension of the aliphatic chain, the isomers that can result from the variation of the position and the number of the methoxyl groups present. and finally those analogs that result from the substitution of a methylenedioxyheterocyclic ring for two adjacent methoxyl groups.
MESCALINE: HOMOLOGS
The aliphatic chain that lies between the aromatic ring system, and the basic nitrogen, for almost all alkaloids in the plant kingdom, is two carbons long. The phenethylamine or indolethylamine (Tryptamine) system is a foundation of nearly all known pharmaceutical agents that are based upon alkaloids. The monomethylene or trimethylene homologs that have been prepared have to a large measure proved to be uninteresting pharmacologically. The most rewarding studies have come from chain lengthenings that have involved homologous families in which the basic nitrogen atom (the amine group) has been maintained in the beta-position (two carbon atoms removed from the aromatic ring). These are the so-called alpha-alkyl homologs such as the phenylisoptopylamines and the phenylsec-butylamines. The simplest of these homologs, the alphamethylphenethylamines, are stimulants of both natural and man-made origins. The compound ephedrine (11) is a component of the plant material that, and is a principle alkaloid in several of the species of Ephedra.

Further, this simple molecular configuration has led to the study of the completely unsubstituted analog, phenylisopropylamine (amphetamine, 12). This latter compound appears to produce a simple sympathomimetic stimulation at least at low dosages, and if there ate any "psychotomimetic" developments they are to be found only at high usage levels which can be achieved only through the development of a tolerance to the normally expected debilitating effects.
The actions of a compound that results from the combination of the features of mescaline and of amphetamine have been described (24). This compound is 3,4,5-trimethoxyamphetamine (3,4,5-trimethoxyphenylisopropylamine, TMA, 13) and it has been established as being an effective sensory distorting agent at levels of about 175 mg (25). It is thus about twice as potent as mescaline.3 An extension of this chain to the 4-carbon homolog (alpha-ethyl- mescaline, 14) produces a compound that lacks all central and peripheral activity in human subjects at acute levels as high as 220 mg (27).
These several chemicals have laid the groundwork for all of the substances to be discussed below, namely that the three carbon chain, the phenyiisopropylamine or amphetamine structure, has proved to be optimum for the generation · of these sensory distortion effects. A decrease of the chain length to two carbons (the loss of the alpha-methyl group) usually retains the nature of activity but with some decrease in potency. A lengthening of the chain to four carbons (the substitution of an alpha-ethyl group) changes the qualitative nature of the pharmacological response.
[ The carbon skeleton end positional location of the ether oxygen atom in 13 (end in MMDA. 25. below) are identical to that found in elemicin end myristicin. components of the spice nutmeg. These Latter nitrogen-free essential cils have recently been shown capable of being converted into the above mentioned amphetamine analogs under biological conditions (26).]
MESCALINE ANALOGS
Variations of the substitution pattern of the methoxyl groups within this generalization of a three-carbon chain optimum, have led to extensive changes in potency. Using 13 as a parent structure, there are five possible positional isomers. These have been synthesized (28) and clinically evaluated in man (29). The first of these positional isomers (2,4,5-trimethoxyphenylisopropylamine, TMA-2, 15) is also related to a series of essential oils, the unconjugated 2,4,5-

trimethoxy- l-allylbenzene found in Caesulia axillaries (30) and the conjugated counterpart in a number of species of Asarum and Acorus (31). The isomer has proven to be the most potent of all six, with an effective dosage level of 20 mg, about a twentieth that required for effective mescaline intoxication. The vicinal analog (2,3,4-tiimethoxyphenylisopropylamine, TMA-3, 16) has neither natural counterparts nor central activity.
The symmetrical isomer (2,4,6-trimethoxyphenylisopropylamine, TMA-6, 17) is without known analogy in the essential oils, but this ring substitution pattern is extremely common throughout the many plant products related to the chromones and the flavonoids. The styles of central activity of 15 and If ate similar to one another in that at threshold levels there is noted only an enjoyable light-headedness coupled with distinct euphoria and a minimum of perceptive distortion. At effective dosages there again is the nausea reminiscent of that seen with mescaline, and the visual distortions can become quite extensive. The sensory disturbances and syntheses so often found to be entertaining or instructive in the case of mescaline intoxication, are found to be disturbing with these latter drugs. TMA-6, If, is about half as potent as 15, thus having some ten times the effectiveness of mescaline. The two remaining possible isomers are known, have been titrated clinically to determine their relative potencies, but have not been explored to an extent adequate for generalizations concerning the qualitative nature of their actions. 2,3,5-Trimethoxyphenyiisopropylamine (TMA-4, 18) is an effective central intoxicant at a 90 mg dosage, and 2,3,6 -trimethoxyphenyli sopropylamine (TMA-5, 19) has similar activity at 30 mg. Both ate thus clearly more potent than mescaline,
A number of isomers of 13 have been studied wherein the variation is with the number of methoxy groups rather than with their position. A single tetramethoxy analog is known, 2,3,4,5- tetramethoxyphenylisopropylamine (20). As with others of the active phenylisopropylamines, there is a corresponding essential oil in nature. This exact carbon skeleton is found in 2,3,4,5-tetramethoxy-l-allyl benzene, one of the major constituents of the seed oil of Apium spp. (32). Compound 20 is effective in man at a dosage of 60 mg, i.e., six times as potent as mescaline (29).

Three of the six possible dimethoxyphenylisopropylamines have been explored pharmacologically. The compound most closely allied with both botanical and mammalian biochemistry is 3,4-dimethoxyphenylisopropylamine (DMA, 21). The plant correlation is to methyleugenol and methylisoeugenol, common essential oils found in a host of plants. In animal chemistry, 3,4-dihydroxyphenethylamine (dopamine) is an intermediate in the synthesis of the transmitter norepinephrine, and- 21 is its trimethyl homolog. The role and action of the two-carbon analog 10 have been discussed above. Compound 21 is slightly less potent than mescaline (33) in that levels of about 700 mg were required parenterally to produce the color syntheses and other sensory distortions ascribed to mescaline. The other two dimethoxy isomers that have been studied, 2,4-dimethoxyphenylisopropylamine (22) and 2,5-dimethoxyphenylisopropylamine (23), are considerably more potent, being active at 70 mg and 45 mg orally, resp. (29).
Only one monomethoxy analog has been explored clinically. In animal studies, this compound (4-methoxyphenylisopropylamine, PMA, 24) is reported to have an hallucinogenic potential parallel to LSD (34).. In man, however, some 75 mg is required (orally) for effective central activity, thus being closer to mescaline than LSD in potency. Arguments that this compound might be an active metabolite of amphetamine in cases of amphetamine-induced psychoses, have been answered by the reported failure to observe this compound in human urine samples following heavy amphetamine administration. It could only be found as an excretory product following its own administration to human subjects (35).
Bases that contain a methylenedioxy ring in place of two adjacent methoxyl groups constitute a large family of compounds that parallel most of the above-mentioned polymethoxylated phenylisopropylamines . In nature, this form of biosynthetic relationship is frequently seen. It occurs within the tetrahydroisoquinoiine alkaloids of peyote itself. A generalized qualitative comment can be made concerning the methylenedioxyphenylisoptopylamines itemized here. In almost every case in which a specific comparison is available between a methylenedioxy compound and its dimethoxy counterpart, the methylenedioxy com pound shows less potential for visual distortion, it is generally more controllable in its extremes of central activity and, as a rule, it is more potent. Of this family only two compounds, 25 and 33, have been sufficiently studied to warrant extended discussion of qualitative subtleties.
Just as there are six possible trimethoxy-substitution isomers of amphetamine, so there are six possible methoxymethylenedioxy isomers. Five of these six have been described chemically and pharmacologically. Their trivial names relate the position of the oxygen substituents directly to the trimethoxy counter part; i.e., MMDA-5 and TMA-5 are both 2,3,6-substitution products. The isomer that is the direct relative of mescaline (1) and the theoretical lophophine (9) is 3-methoxy-4,5-methyienedioxyphenyiisopro (MMDAI 25). The carbon skeleton in this instance is that of myristicin and isomyristicin, essential oils found throughout the plant kingdom. This compound was synthesized and evaluated independently in two separate laboratories (36, 37). The compound is active in man at 130 mg, and so has nearly three times the potency of mescaline. Unlike mescaline, however, its actions are largely free of perceptual distortions, and seem not to inflexibly command the complete attention of the subject. There is an element of choice in the degree of compliance to the intoxication, and there seems to be some valid access to memory material. These properties have been discussed in a recent monograph (38). The compound analogous to TMA-2 (15) is 2-methoxy-4,5-methylenedioxyphenylisopropylamine (MMDA-2, 26). An essential oil (carpacin) with this substitution pattern has been isolated from Cinnamomumspp. (39).

The base 26 is only slightly less active in human trials (30 mg) than the trimethoxy counterpart. Two possible 2,3,4- trisubstituted compounds can exist that correspond to 16. These are 2-methoxy-3,4-methylenedioxyphenylisopro (MMDA- 39, 21) and 4-methoxy-2,3 -methylenedioxyphenyiisopropylamine (MMDA-3b, 28). The first of these has a natural counterpart in the ether croweacin isolated from Eriostemon crowei (40); the second has no known analogy in nature. Although the trimethoxy compound with this vicinal substitution pattern was not active, both of these compounds have been shown to be effective intoxicants, the first at a 35 mg dose (41) and the second at 120 mg (29). The fifth of these methoxy methylenedioxy isomers is 6-mtthoxy-2,3-methylentdioxyphenylisopropylamine (MMDA-5, 30) which corresponds exactly to TMA-5 (19) both in substitution pattern and in potency (42). No successful synthesis yet exists for 5-methoxy-2,3-methylenedioxvphenylisopro (MMDA-4, 29). The analog to TMA-6 (lit) cannot of course exist. Only three methylenedioxy compounds that are not trisubstituted have beer studied in man. Two of these are the tetrasubstituted analogs of apiole and dill-apiole, both well explored essential oils from a number of spices.

These are 2,5 -dimethoxy-3,4-methylenedioxyphenyiisopr (31) and 2,3-di-methoxy-4 ,S-methylenedioxyphenyiisopropylamine (.32). They are active at 30 mg and 70 mg resp. (43) corresponding to twelve times, and five times the potency of mescaline. The -remaining compound is 3,4-methylenedioxyphenyl isopropylamine (MDA, 33) which, along with MMDA (25) is the only material sufficiently well studied to warrant generalizations concerning comparative qualitative properties (44). Although this compound is not particularly potent (typical dosage, some 120 mg or about three times the activity of mescaline) its easy availability and apparent consistency of action have led to its study in a number of laboratories. The 2,3-isomer is unknown.
The last group to be discussed consists of compounds that are quite removed from nature. They have evolved quite logically however from the importance that is clearly assignable to the 4-position of the aromatic substitution pattern. An example is that, of all the several possible ethoxy homologs of the maximally potent trimethoxy-compound 15, it is only the 4-ethoxy compound (2,5 -dimethoxy-4-ethoxyphenylisopropylamine, MEM, 3r) that maintains complete central activity at the same potency (45). An argument can be extended that this positional sensitivity might be associated with a theoretical ease of metabolic attack at this position. Consistent with this approach, one could expect that the replacement of an easily attackable group (alkoxy) with one less amenable to removal (aikyl) might radically affect the pharmacological properties of the corresponding amine. The simplest such example, 2,5-di-methoxy-4-methylphenylisoptopylamine (DOM, STP, 35)

proved to be several times more potent than 15 as a centrally active psychotomimetic. With an effective, not threshold, level of activity of 3 mg total dosage, it has over one hundred times the potency of mescaline. Further, the nature and chronology of the intoxication is quite changed from that common to most of these chemicals discussed. Most noteworthy is the unexpected lengthening of the time course by a factor of three or more. Several clinical studies have appeared in the medical literature concerning this drug (46, 47). The 4-ethyl homolog (2,5-dimethoxy-4-ethyiphenylisopropylamine, DOET, 36) appears to be fully as potent as DOM (35) although at lower clinical levels the psychological changes noted seem to be somewhat more controllable and thus more constructive (118). Recently yet another analog of this system has been described. In this, the 4-methyl group has been replaced with a bromo substituent to produce 2,5dimethoxy-4-bromophenylisopropylamine (37) (49, 50). This compound has in man an effective response level of between 0.5 and 1.0 mg, thus it is several hundred times more potent than mescaline. Yet in the qualitative sense it presents more of the clinical picture that has been associated with MDA (33) (49). A positional isomer has been described (2,4-dimethoxy-5-bromophenylisopropylamine, 38) which is much less potent than 37 (100 mg for an effective intoxication) but which maintains the MDA-like response is1>. The 2-bromoanalog of MMDA-2 (2-bromo-4,5-methylenedioxy·phenylisopropolymine) is less active than mescaline (>350 mg in man) and seems to produce an amphetamine-like response.
A final comment is in order concerning the frequent use of the term "amphetamines" to describe these compounds. With the exception of some of the peyote components, all of the centrally active substances that have been discussed here contain the carbon skeleton of the amphetamine molecule. Yet almost none of them display the sympathomimetic stimulation that is the reputation of amphetamine. The popular use of the term "amphetamines" has come to embrace a large number of currently used drugs that are stimulants, and which call upon these effects for their effectiveness. Examples of this are amphetamine itself (and its dextrorotatory isomer, dexedrine), methamphetamine, methylphenidate (Ritalin), phenmetrazine (Preludin) and mephenteramine (Wyamine). An excellent demonstration of the separation of these pharmacological areas may be seen from the fact that the active isomer of DOM (35) is the R (-) form. The S (+) form shows only stimulant properties, and this is the same absolute configuration as that seen in the stimulatively active isomer of amphetamine itself, S-(+) -dextroamphetamine (52).
This has been an up-to-date presentation of our knowledge of the structural requirements for hallucinogenesis (or whatever this form of central stimulation and sensory modification might be called). At this state of chemical evolution, the structure of the parent compound mescaline might well look quite foreign. However, its role has been evident as a starting point in this rapidly evolving area of psychopharmacology.